
Palladium-Catalyzed Enantioselective C-3 Allylation of 3-Substituted-1H- Indoles Using Trialkylboranes | Journal of the American Chemical Society

Palladium-Catalyzed Enantioselective C-3 Allylation of 3-Substituted-1H- Indoles Using Trialkylboranes | Journal of the American Chemical Society

Molecules | Free Full-Text | Recent Advances in the Synthesis of 3,n-Fused Tricyclic Indole Skeletons via Palladium-Catalyzed Domino Reactions

Palladium-catalyzed regio- and enantioselective migratory allylic C(sp3)-H functionalization | Nature Communications

Synthesis of 2,3-Disubstituted Indoles via Palladium-Catalyzed Annulation of Internal Alkynes | The Journal of Organic Chemistry

Palladium-Catalyzed Asymmetric Allylic Alkylation/α-Iminol Rearrangement: A Facile Access to 2-Spirocyclic-Indoline Derivatives | CCS Chemistry

Pd-Catalyzed C3-Selective Allylation of Indoles with Allyl Alcohols Promoted by Triethylborane | Journal of the American Chemical Society

Application of the Asymmetric Pictet-Spengler Reaction in the Total Synthesis of Natural Products and Relevant Biologically Active Compounds. - Abstract - Europe PMC

The Palladium Catalyzed Asymmetric Addition of Oxindoles and Allenes: An Atom-Economical Versatile Method for the Construction of Chiral Indole Alkaloids | Journal of the American Chemical Society
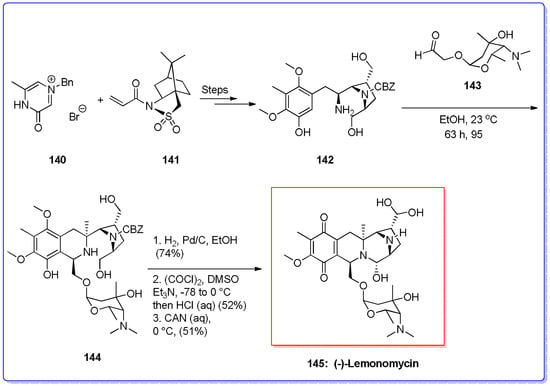
Molecules | Free Full-Text | Application of the Asymmetric Pictet–Spengler Reaction in the Total Synthesis of Natural Products and Relevant Biologically Active Compounds

Synthesis of 2,3-Disubstituted Indoles via Palladium-Catalyzed Annulation of Internal Alkynes | The Journal of Organic Chemistry

Molecules | Free Full-Text | Application of the Asymmetric Pictet–Spengler Reaction in the Total Synthesis of Natural Products and Relevant Biologically Active Compounds

Synthesis of 3-acylindoles by palladium-catalyzed acylation of free (N-H) indoles with nitriles. | Semantic Scholar

Molecules | Free Full-Text | Application of the Asymmetric Pictet–Spengler Reaction in the Total Synthesis of Natural Products and Relevant Biologically Active Compounds

Application of the Asymmetric Pictet-Spengler Reaction in the Total Synthesis of Natural Products and Relevant Biologically Active Compounds. - Abstract - Europe PMC

Application of the Asymmetric Pictet-Spengler Reaction in the Total Synthesis of Natural Products and Relevant Biologically Active Compounds. - Abstract - Europe PMC

Synthesis of 2,3-Disubstituted Indoles via Palladium-Catalyzed Annulation of Internal Alkynes | The Journal of Organic Chemistry
![Palladium-catalyzed carbonylative cyclization of alkene-tethered indoles with phenols or arylboronic acids: Construction of carbonyl-containing indolo[2,1-a]isoquinoline derivatives Palladium-catalyzed carbonylative cyclization of alkene-tethered indoles with phenols or arylboronic acids: Construction of carbonyl-containing indolo[2,1-a]isoquinoline derivatives](http://www.ccspublishing.org.cn/fileHXH/journal/article/ccl/2023/5/PIC/ccl-34-5-107873-1-S4.jpg)
Palladium-catalyzed carbonylative cyclization of alkene-tethered indoles with phenols or arylboronic acids: Construction of carbonyl-containing indolo[2,1-a]isoquinoline derivatives

PDF) Application of the Asymmetric Pictet–Spengler Reaction in the Total Synthesis of Natural Products and Relevant Biologically Active Compounds

Molecules | Free Full-Text | Application of the Asymmetric Pictet–Spengler Reaction in the Total Synthesis of Natural Products and Relevant Biologically Active Compounds
![Catalytic asymmetric dipolar cycloadditions of indolyl delocalized metal-allyl species for the enantioselective synthesis of cyclopenta [b] indoles and pyrrolo[1,2-a]indoles | Science China Chemistry Catalytic asymmetric dipolar cycloadditions of indolyl delocalized metal-allyl species for the enantioselective synthesis of cyclopenta [b] indoles and pyrrolo[1,2-a]indoles | Science China Chemistry](https://media.springernature.com/lw685/springer-static/image/art%3A10.1007%2Fs11426-020-9854-3/MediaObjects/11426_2020_9854_Fig1_HTML.jpg)


