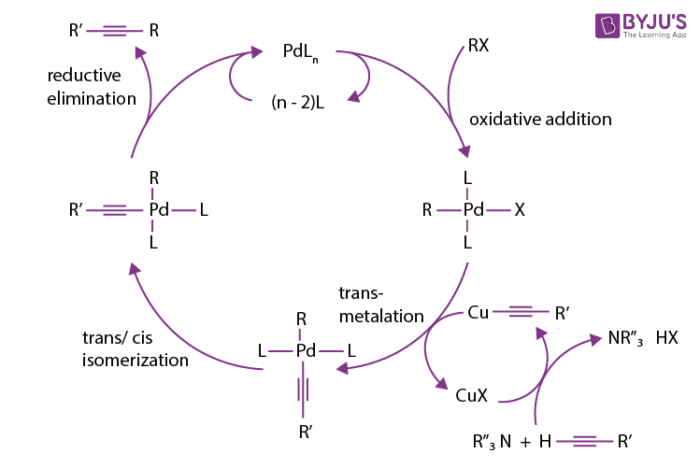
Palladium-Catalyzed Carbothiolation of Alkenes and Alkynes for the Synthesis of Heterocycles | ACS Catalysis

Palladium–Protein Oxidative Addition Complexes by Amine-Selective Acylation | Journal of the American Chemical Society

Different Oxidative Addition Mechanisms for 12- and 14-Electron Palladium(0) Explain Ligand-Controlled Divergent Site Selectivity | ACS Catalysis
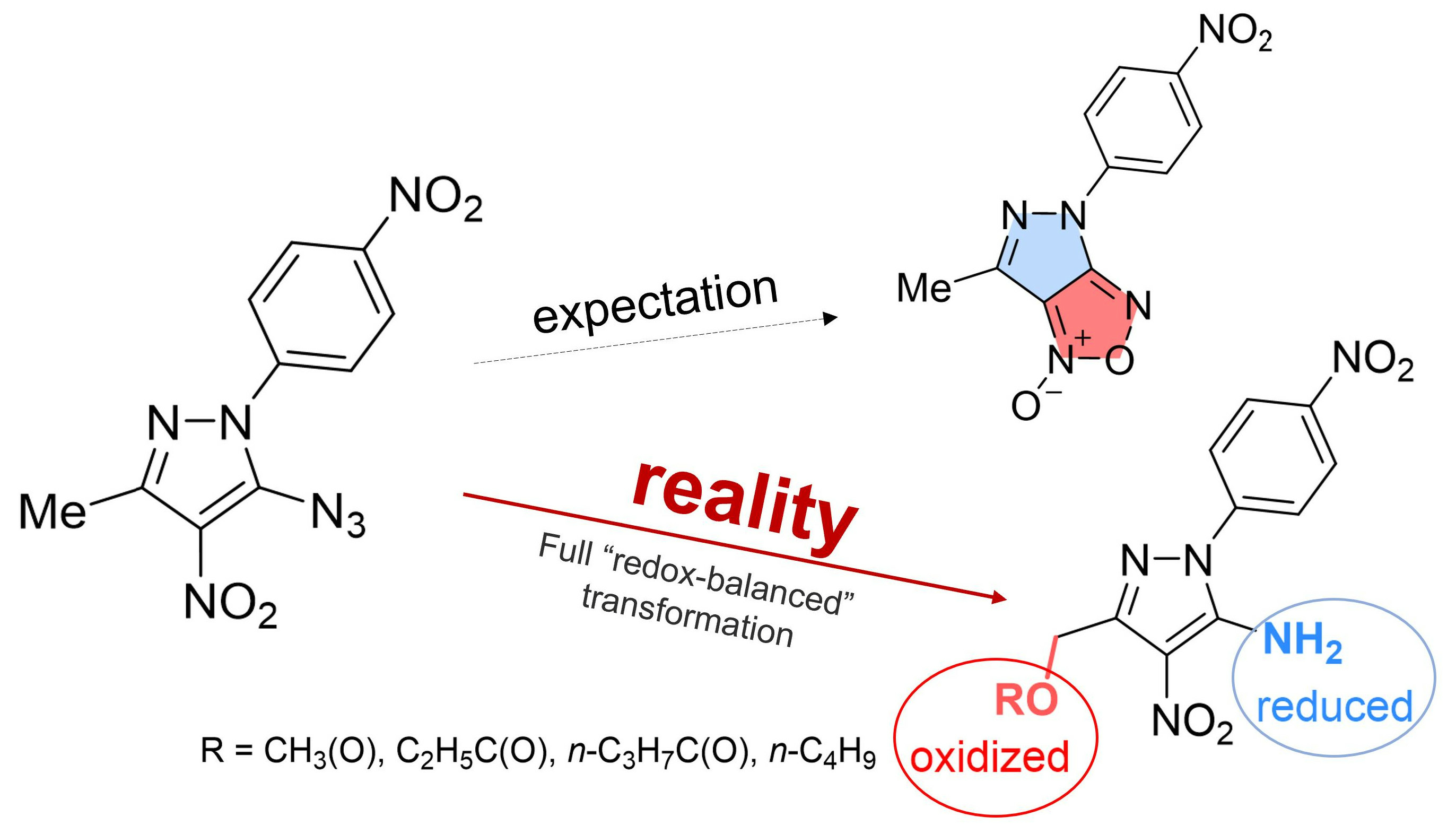
Molecules | Free Full-Text | An Unusual Rearrangement of Pyrazole Nitrene and Coarctate Ring-Opening/Recyclization Cascade: Formal CH–Acetoxylation and Azide/Amine Conversion without External Oxidants and Reductants

Oxidative Addition to Palladium(0) Made Easy through Photoexcited‐State Metal Catalysis: Experiment and Computation - Kancherla - 2019 - Angewandte Chemie International Edition - Wiley Online Library

Palladium Oxidative Addition Complexes for Peptide and Protein Cross-linking | Journal of the American Chemical Society

Palladium–Protein Oxidative Addition Complexes by Amine-Selective Acylation | Journal of the American Chemical Society

The mechanism of oxidative addition of Pd(0) to Si–H bonds: electronic effects, reaction mechanism, and hydrosilylation - Chemical Science (RSC Publishing) DOI:10.1039/D1SC04419B
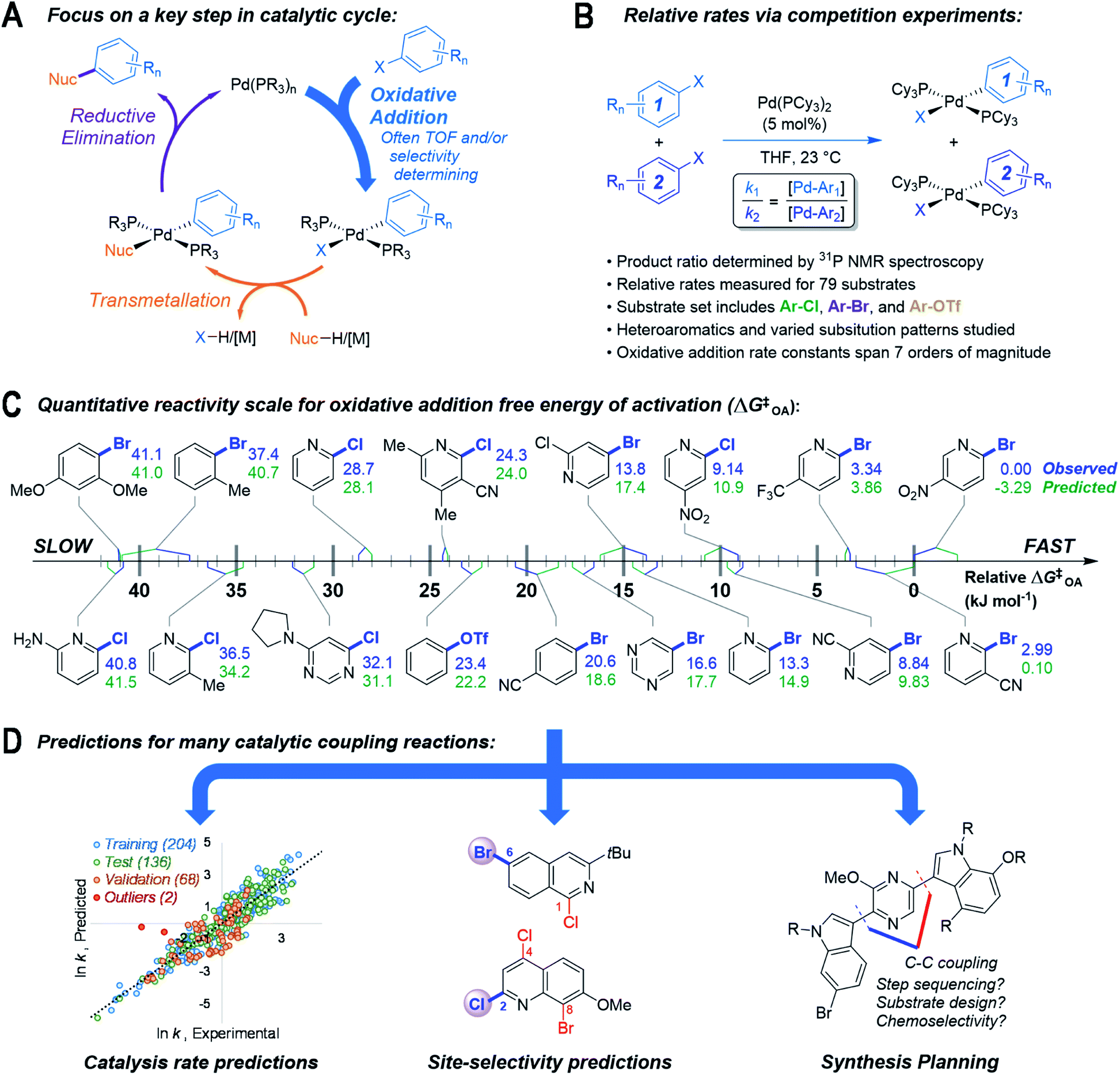
A reactivity model for oxidative addition to palladium enables quantitative predictions for catalytic cross-coupling reactions - Chemical Science (RSC Publishing) DOI:10.1039/D2SC00174H

Arylic C–X Bond Activation by Palladium Catalysts: Activation Strain Analyses of Reactivity Trends | Scientific Reports

Report: Toward Greater Understanding and Expanded Utility of the Palladium-Catalyzed Activation of Carbon-Carbon Single Bonds (56th Annual Report on Research Under Sponsorship of The American Chemical Society Petroleum Research Fund)